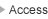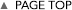Research Theme 2
Biology of protein lysine methylation
The modulation of protein-protein interaction is a key regulatory mechanism for various signaling cascade pathways that control most biological processes. Modulation of intracellular localization or conformational changes in target molecules is crucial for such regulation. In many cases, post-translational modifications play an important role in the regulation of these pathways. For example, phosphorylation can induce conformational changes in target molecules or create binding sites for the interacting molecules.
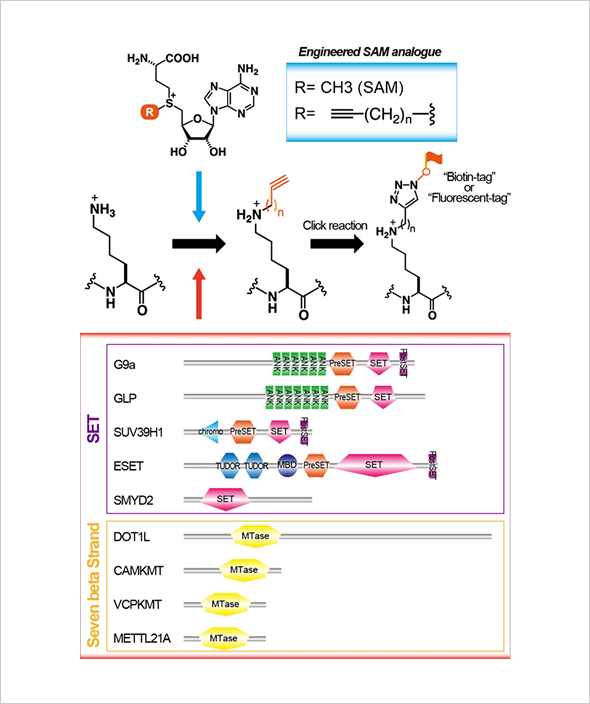
Recently, many enzymes involved in protein lysine methylation have been discovered in the animal kingdom. They are classified as SET-domain containing proteins and methylate-specific lysine residue(s) of histone molecules. From the studies of SET-domain enzymes, histone lysine methylation has been shown to play many important roles in chromatin-based biological processes, including gene expression, DNA repair, replication, and chromatin condensation. Methylation of specific histone lysine residue(s) activates unique biological process(es) by recruiting different functional molecule(s) to chromatin loci containing different histone methyl mark(s). Thus, histone lysine methylation also controls protein-protein interactions. A major reason for the dominated studies of lysine methylation in chromatin function is that SET-domain molecules may have evolved as special enzymes for histone molecules. However, it is not surprising that lysine methylation occurs on non-histone proteins and those methylations also play crucial roles for their regulation since other posttranslational modifications occur on various different protein molecules and regulate their functions. Additionally, non-SET-domain lysine methyltransferases, known as seven beta-strand enzymes/"Class I" methyltransferases, have recently been identified, and they target non-histone proteins. We would like to establish a novel detection system for protein lysine methylation by using synthetic cofactors as methyl donors. By using this system, we will elucidate novel roles of lysine methylation (especially non-histone methylation) in higher-order biological processes.